Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)
Advancing Gene Editing with Precision and Prudence
CRISPR technology, with its unparalleled ability to edit the very blueprint of life, has positioned itself at the cutting edge of scientific innovation, promising profound progress and raising equally profound questions. The story of He Jiankui, the Chinese scientist who ventured into the world of genetic engineering, brought gene editing into mainstream consciousness, with ethical quakes that rippled through the field. Jiankui's covert experimentation led to a watershed moment: the birth of the first genetically edited humans. This act, which earned him a three-year prison sentence in 2019, symbolized a crossing of the Rubicon, sparking a worldwide discourse at the murky crossroads of morality and science.
CRISPR technology revolutionized genetic engineering with the realization that it can create precise breaks in both strands of DNA in mammalian cells. By co-opting natural DNA repair processes, CRISPR systems can facilitate the repair of deficient genes with relative ease and unprecedented precision. The practical applications of CRISPR are vast, ranging from rescuing disease phenotypes to the development of highly sensitive and specific “biosensing” diagnostics.
However, the path forward is not without complexity. Most genetic disorders do not arise from single-gene anomalies but from intricate combinations of mutations, deletions, and duplications throughout the genome, necessitating sophisticated engineering strategies that go beyond simple gene knockouts and manipulations. In response to these intricacies and the technical challenges they present, efforts are underway to broaden the capabilities of CRISPR, enabling more nuanced and precise forms of gene editing tools. As a result, the horizon of CRISPR's potential is expanding, not just in rewriting genes, but in rewriting the narrative of human health and disease at the level of single nucleotides.
Background
In the early 2000's, scientists encountered repetitive DNA segments in the genome of the bacterium Escherichia coli. These segments were later named clustered regularly interspaced short palindromic repeats (CRISPR) and, interestingly, they corresponded to the DNA of viruses known as phages that infect bacteria.
Adjacent to the CRISPR sequences, researchers identified a set of genes encoding proteins, later named CRISPR-associated (Cas) proteins. These proteins, particularly Cas9, were soon understood to be the executors of a bacterial defense strategy (adaptive immunity) against phage attacks. The process begins when the CRISPR regions are transcribed into long RNA molecules. These are then sliced into smaller fragments, each containing a spacer sequence—a direct copy of a phage DNA sequence. This spacer-carrying RNA, termed CRISPR RNA (crRNA), teams up with another RNA, the trans-activating CRISPR RNA (tracrRNA). Together, they form a complex with the Cas9 protein. This complex patrols the bacterial cell, hunting for invading phage DNA.
When the crRNA spacer finds its counterpart sequence in the phage DNA, it binds to it, guiding Cas9 to the precise spot. Cas9 then slices the DNA, creating double-strand breaks, thus neutralizing the phage's ability to replicate and saving the bacterium from the viral onslaught. This elegant mechanism not only provides a defense against immediate threats but also allows the bacterium to "remember" the phage, as new spacers are incorporated into the CRISPR array following an encounter, providing immunity against future attacks.
The revelation that bacteria have a mechanism for adaptive immunity similar to that of mammals was groundbreaking. It opened up the possibility that other forms of life might also have unexpected ways of dealing with pathogens. Furthermore, the way bacteria employed the CRISPR-Cas9 system to disable phage DNA was quickly recognized as a tool that could be repurposed for genome editing in more complex organisms, including humans, with profound implications for medicine and biotechnology.
The rest, as they say, is history with some surprises along the way.
CRISPR targeting of specific human genes is typically engineered today through a multi-step process that involves designing, constructing, and delivering the components of the CRISPR system into human cells.
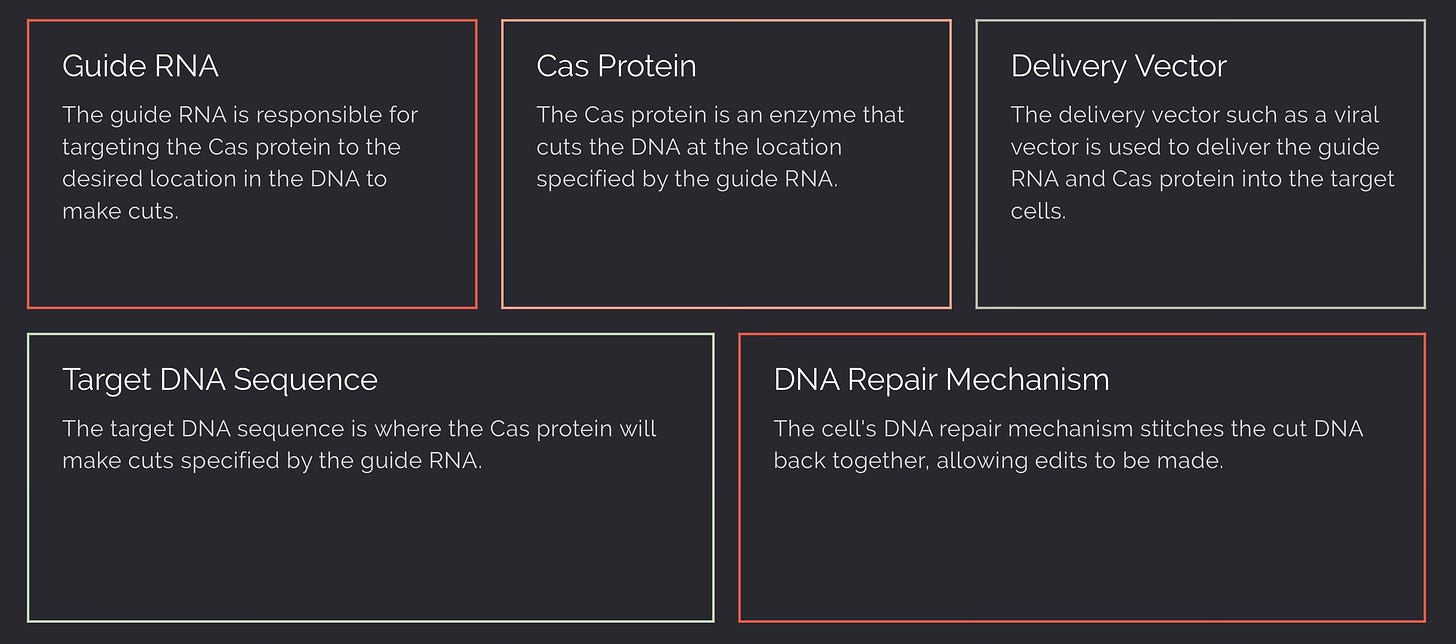
1. Designing Guide RNA (gRNA)
- The gRNA is synthesized to include a region of about 20 base pairs that is complementary to the target DNA sequence. This allows the gRNA to direct the Cas protein to the exact location in the genome where an edit is desired.
2. Cas Protein Selection
- The Cas protein is chosen based on its cutting efficiency, specificity, and other factors such as the size of the protein, which can affect delivery into cells.
3. Vector Construction
- The DNA sequences encoding the gRNA and the Cas protein are inserted into a plasmid or viral vector. This vector will carry the CRISPR components into the human cells. Sometimes, additional elements such as promoters, which drive the expression of the Cas protein and gRNA, or selection markers, which help identify cells that have taken up the CRISPR components, are also included in the vector.
4. Delivery into Human Cells
- The vectors containing the CRISPR components are delivered into human cells through various methods such as electroporation (creating temporary pores in cell membranes), lipid nanoparticles, viral vectors, or physical injection. Once inside the cell, the Cas protein and gRNA form a complex that locates the target DNA sequence.
5. DNA Targeting and Cleavage
- The gRNA guides the Cas protein to the target DNA sequence through base pairing. Once the target DNA is bound by the gRNA-Cas complex, the Cas protein creates a double-strand break at that specific location.
6. DNA Repair and Gene Editing
- The cell's natural DNA repair mechanisms then repair the break. This can be harnessed to disrupt a gene (by non-homologous end joining, which can introduce errors) or to insert a new DNA sequence (by homology-directed repair, which uses a supplied DNA template with the desired sequence).
7. Verification
- After the CRISPR system has been applied, verification can be done using techniques such as PCR amplification and sequencing, or by assessing the phenotype of the edited cells.
8. Optimization and Control
- To minimize off-target effects, the gRNA design and CRISPR system delivery are often optimized. This can involve using high-fidelity Cas variants, modifying the gRNA structure, or regulating the duration of Cas protein activity.
Current Landscape
There are several distinct Cas protein families with various classes and subtypes. Most recently, the discovery of CasX has illustrated the dynamic nature of CRISPR-Cas systems. CasX, also called Cas12e, is a compact and distinct subtype within the broader classification of Class 2 Type V CRISPR-Cas effectors. The Class 2 CRISPR-Cas systems are characterized by their use of a single RNA-guided protein for DNA targeting and cleavage, distinguishing them from Class 1 systems which employ multiprotein complexes for the same purpose. CasX's uniqueness lies in its compact size, with less than 1000 amino acids, which contrasts with other more commonly known Class 2 effectors such as Cas9. The compact nature of CasX provides an advantageous framework for gene editing, particularly when size constraints are a factor. Scribe Therapeutics has developed a platform called "Crispr-by-design" using CasX proteins. This platform is designed for delivering gene editing therapies directly to patients "in vivo" rather than removing and editing cells outside the body.
Earlier this year, Vertex and CRISPR Therapeutics submitted their CRISPR-based ex vivo cell therapy, exagamglogene autotemcel (exa-cel) for FDA approval for sickle cell disease (SCD) and beta-thalassemia. The companies have also filed for approval in Europe and the UK. Recently, the FDA advisory committee reviewed exa-cel, with special focus on evaluating the "off-target" effects of treatment, where unintended edits may occur in the DNA. While the committee acknowledged uncertainties and unknowns regarding off-target effects, they did not question the importance of the treatment. The FDA will make a decision by December 8 on the approval of exa-cel for the treatment of patients with SCD. Additionally, the FDA is expected to reach a verdict for exa-cel in beta-thalassemia by March 30, 2024.
Challenges and Future Directions
A key challenge in the development of CRISPR-based treatments is the potential for off-target effects, where the CRISPR system might inadvertently alter genetic sequences beyond the specified target, potentially resulting in adverse outcomes. Advancing the precision in editing specificity not only involves refining the core editing mechanisms but also necessitates meticulously designed clinical trials and postmark surveillance strategies that include extensive long-term monitoring. Such rigorous approaches are essential to bolster the therapeutic applications of CRISPR and to assure the overall safety and efficacy of gene-editing interventions in clinical practice.
The delivery of CRISPR components to specific cells for therapeutic applications hinges on the development of reliable delivery methods. While a range of delivery mechanisms is under investigation, the targeted and efficient transport to designated cell types or tissues remains highly nuanced undertaking. The physical limitations imposed by the size of CRISPR payload can further complicate delivery. The need to transport multiple elements, such as the guide RNA and Cas protein, introduces size restrictions that may impact therapeutic delivery and integration into viral vectors as primary mode of delivery, prompting the need for creative resolutions to these constraints. Furthermore, the potential immune reaction against CRISPR components can be problematic. The immune system's recognition of CRISPR elements as exogenous could elicit reactions that may diminish treatment efficacy or provoke adverse reactions.
Machine learning (ML) and artificial intelligence (AI) are starting to be applied to various aspects of CRISPR technology to address some of the challenges. ML methods have been used to optimize the CRISPR/Cas9 system by improving on-target efficiency, reducing off-target effects, and enhancing sgRNA design. These methods include predicting CRISPR gRNA on- and off-target activities using as well as evaluating CRISPR genome-edited data generated through Next Generation Sequencing platforms. Additionally, AI approaches have been used in combination with CRISPR/Cas9 for tasks such antigen and epitope prediction. The use of AI and ML in CRISPR technology remains at its early stages but has the potential to improve the efficiency and specificity of gene editing while broadening its applications in cancer treatment, vaccine design, and the development of CRISPR biosensors.
CRISPR biosensors have gained significant attention in recent years due to their potential applications in disease detection and monitoring. These biosensors utilize the CRISPR-Cas system to detect and bind to specific DNA or RNA sequences, which can theoretically enable highly sensitive and specific detection. The CRISPR-Cas system has been used for the detection of miRNAs, which are important post-transcriptional regulators implicated in diseases like cancer. These biosensors have the potential for point-of-care testing, which can power rapid and field-deployable disease detection. They can also be combined with amplification technologies like PCR to enhance sensitivity and selectivity. Future directions include developing multiplex assays in addition to identification of commercially viable applications of CRISPR detection technology compatible with existing clinical workflows and third-party reimbursement practices.
As the controversy surrounding Jiankui’s clandestine experiments illustrates, the journey of CRISPR technology underscores the delicate interplay between scientific possibility and ethical responsibility. Ethical considerations, particularly with regards to CRISPR-guided germline editing, which carries the potential for heritable genetic alterations, are critically important. The profound implications of such modifications demand extensive discourse and robust regulatory oversight. It is, therefore, essential that regulatory frameworks be anticipatory, evolving in tandem with, or even ahead of, scientific discoveries to ensure responsible stewardship of gene-editing technologies. These measures would not only safeguard against unforeseen consequences but also maintain public trust and uphold ethical standards in biomedical research and therapeutic development. As CRISPR research continues to progress swiftly, it is vital to recognize and tackle such issues to pave the way for broader clinical application. The synthesis of our expanding knowledge, technological breakthroughs, and ethical governance is imperative to harness the full capabilities of CRISPR responsibly. This integration would weigh the potential benefits against the risks inherent in gene editing, especially considering our nascent, albeit rapidly evolving, understanding of the human genome. Ensuring that this balance is maintained is key to fully harnessing the power of CRISPR technology and advancing the field.
References
CRISPR/Cas9 therapeutics: progress and prospects. (2023). Signal Transduction and Targeted Therapy, 8, Article number: 36.
Zeng, L., et al. (2022). Broad-spectrum CRISPR-mediated inhibition of SARS-CoV-2 variants and endemic coronaviruses in vitro. Nature Communications, 13, 2766.
Raguram, A., Banskota, S., & Liu, D. R. (2022). Therapeutic in vivo delivery of gene editing agents. Cell, 185(12), 2806–2827.
CRISPR in cancer biology and therapy. (2022). Nature Reviews Cancer, 22, 259–279.
Nidhi, S., Anand, U., Oleksak, P., Tripathi, P., Lal, J., Thomas, G., … & Tripathi, V. (2021). Novel crispr–cas systems: An updated review of the current achievements, applications, and future research perspectives. International Journal of Molecular Sciences, 22(7), 3327.
Ntui, V., Tripathi, J., & Kumar, P. (2021). Application of CRISPR/Cas for diagnosis and management of viral diseases of banana. Frontiers in Microbiology, 11.
Maserat, E. (2021). Integration of Artificial Intelligence and CRISPR/Cas9 System for Vaccine Design. Cancer Informatics, 21.
Potts, M., McDonald, J., Sutherland, K., & Herold, M. (2020). Critical cancer vulnerabilities identified by unbiased CRISPR/Cas9 screens inform on efficient cancer immunotherapy. European Journal of Immunology, 50(12), 1871-1884.
Xu, Y., & Li, Z. (2020). CRISPR-Cas systems: Overview, innovations and applications in human disease research and gene therapy. Computational and Structural Biotechnology Journal, 18, 2401-2415.
Zhang, B. (2020). CRISPR/Cas gene therapy. Journal of Cellular Physiology, 236(4), 2459-2481.
Wang, J., Zhang, X., Cheng, L., & Luo, Y. (2019). An overview and metanalysis of machine and deep learning-based CRISPR gRNA design tools. RNA Biology, 16(10), 13–22.
Choudhary, S., et al. (2019). Application of Bioinformatics Tools in CRISPR/Cas. In Bioinformatics (pp. 19-38). Springer, Cham.
Zhang, G. S., et al. (2018). Application of machine learning in the CRISPR/Cas9 system. Yi Chuan, 40(9), 704–723.
Doudna, J., & Charpentier, E. (2014). The new frontier of genome engineering with CRISPR-cas9. Science, 346(6213).